Chitosan in bio-based food contact materials - Annex B is reserved
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Chitosan in bio-based food contact materials - Annex B is reserved
Introduction
1. In May 2020, a scoping paper entitled “Alternatives to conventional plastics for food & drinks packaging” (TOX/2020/24), which introduced some of the possible toxicological hazards associated with the use of bio-based food contact materials (BBFCMs), was presented to the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT). Subsequently, a proposed list of BBFCMs for health risk assessment was presented to the COT in February 2021 (TOX/2021/01); this included BBFCMs containing chitosan.
2. A first draft statement on the potential allergenicity of chitosan in food contact materials (FCMs) was presented to the COT on 7th September 2021. At this meeting, additional information was requested by the Committee on the following:
- life-cycle assessments (LCA) for bio-based materials versus conventional plastics;
- information from the UK Medicines and Healthcare products Regulatory Agency (MHRA) on any reports of adverse events following dermal exposure to chitosan in medical devices;
- any reports of adverse health effects reported in the scientific literature from consumption of culinary dishes that include crustacean shells;
- further information on product types which carry risk management or warning labels;
- information on the biological source of the chitosan; and,
- an estimation of total exposures to allergenic proteins in BBFCMs in contact with different foods.
The information obtained has been presented below.
Life-cycle assessments (LCAs)
3. Life cycle assessment (LCA) has been defined as “a technique to evaluate the environmental aspects related to a product, where the product is followed in quantitative terms from raw materials extraction through production and use, to its disposal” (Baumann & Tillman, 2004; Leceta et al., 2013).
4. In their discussion of LCA of chitosan as edible coatings and films, Ghosh & Katiyar (2020) note that: “bio-based products have less impact on the environment in comparison to fossil-based products”. Several studies were referenced in respect of this: Madival et al. (2009), Leceta et al. (2013), and Suwanmanee & Lertworasirikul (2015).
5. Madival et al. (2009) used computer software (SimaProTM, developed by PRé Sustainability) to assess the environmental profile of strawberry containers using LCA methodology. The strawberry containers were made of poly(lactic acid) (PLA), poly(ethylene terephthalate) (PET), or poly(styrene) (PS). PLA is a bio-based material, whereas PET and PS are conventional plastics. For PLA, the LCA included various inputs (such as fertilisers and pesticides used in corn farms), whereas for PET and PS, other processes were considered (extraction and refinement of crude oil). The impact categories assessed were: global warming, aquatic acidification, aquatic eutrophication, aquatic ecotoxicity, ozone depletion, non-renewable energy and respiratory organics, land occupation and respiratory inorganics. The geographical scope of the study reflected data from Europe, North America and the Middle East. The total normalised impact values for strawberry containers made of PLA, PET, or PS were 10.3, 11, and 9.8, respectively. The study authors noted that “PET contributed the highest in almost all the impact categories. This could be largely attributed to the higher weight of the(se) containers” and “the longest transportation distance of the resin”. However, PLA scored highest in the following sub-categories: aquatic acidification, respiratory organics, and respiratory inorganics.
6. Leceta et al. (2013) compared the environmental impacts of shrimp-derived chitosan based films with those of conventional polypropylene (PP) packaging in different stages of their life cycles. Computer software (Eco-indicator 99, developed by PRé Sustainability) was used to generate normalised impact values for a number of categories (i.e. carcinogens, respiratory organics, respiratory inorganics, climate change, radiation, ozone layer, ecotoxicity, acidification/eutrophication, land use, minerals, and fossil fuels). The results showed that chitosan-based films caused higher environmental damage than PP films in three categories: respiratory inorganics and minerals (which Leceta et al. attributed to the use of hydrochloric acid in production of chitosan films), and land use (attributed to use of glycerine as a plasticiser in production of chitosan films). On the other hand, PP films had significantly higher impacts than chitosan-based films in two categories: carcinogens (attributed to harmful emissions into atmosphere, groundwater, and surface water from landfill sites) and fossil fuels (attributed to energy required to extract fossil fuels). Overall, Leceta et al. concluded that the “composting scenario for chitosan-based films exhibits a highly positive effect on the environment in comparison with the end of life scenarios for PP films, providing the chance of reducing the environmental pollution generated by the food packaging industry on disposal”.
7. Suwanmanee & Lertworasirikul (2015) used computer software (SimaProTM, developed by PRé Sustainability) to assess the environmental impacts associated with coating fresh-cut papaya with a chitosan solution. Suwanmanee & Lertworasirikul concluded that “from the LCA analysis of coating fresh-cut papaya with 2% chitosan solution, the highest impact occurred during the production. The main environmental impacts were marine aquatic ecotoxicity, global warming and human toxicity, respectively. An essential factor which caused these impacts was the usage of electricity during production process”.
UK MHRA
8. The UK MHRA is aware of chitin and chitosan being used in medical devices, but is not aware of any safety issues related to this material that have come to light since receiving market authorisation. This relates to medical devices on the UK market only. However, if a safety signal is identified in the future relating to the use of chitosan in medical devices, the MHRA will initiate an investigation.
9. The MHRA has not conducted a risk assessment on the use of chitosan in medical devices as they do not hold information on the material or chemical make-up of medical devices (it is not within the remit of MHRA to do so). A manufacturer is required to conduct an assessment of the biological risks before a device can be placed on the market, including the risks posed by the materials and chemicals used to manufacture the device. Medical devices are granted market approval and regulated through an independent third-party organisation called an ‘Approved Body’, which assesses the manufacturer to ensure they are following the medical devices regulations. The MHRA audits Approved Bodies in the UK to ensure they are fulfilling their obligations.
10. The MHRA has had two reported events of allergy in relation to medical devices containing chitosan (i.e. wound dressings) in the last 5 years (as of June, 2022). The two manufacturers of these wound dressings were contacted by the MHRA for further information, however as the manufacturers no longer market these devices in the UK, they were not forthcoming to the MHRA with any information regarding the formulation.
Crustacean shells used in cooking
11. There are several instances where people could be exposed to crustacean shells without the meat. For example, when crustacean shells are used in cooking to make shellfish stock, primarily to enhance flavour. Another example is in a shell-handling factory. Several studies on these aspects are described below.
12. Nguyen (2012) used an indirect ELISA method to estimate tropomyosin concentrations in tail and shell extracts of shrimp, which were reported to be “approximately 3.5 μg/mL and 1.0 μg/mL, respectively” (p.91).
13. Additionally, Nagano et al. (1984) used the radioallergosorbent test (RAST) to confirm three cases of allergic contact urticaria from the shells (alone) of raw prawns (n = 2) and shrimps (n = 1) in two patients with a history of urticaria, and considered this reaction to be “rare” in the population.
14. Kim et al. (1982) surveyed 26 employees of a shell-handling factory on whom skin tests with shell powder extract (SPE) were done. Positive skin responders to SPE were among 8 of the 26 subjects (30.8 %). Among the 8 subjects who had a positive skin response, 4 also had respiratory symptoms (one case of early, one late, and one dual bronchial responses were observed). The cases of positive skin responses to SPE were noticed after 2 months of employment, whilst the cases of respiratory symptoms developed after 3 months.
Risk management and warning labels
15. The COT requested further information on other similar product types which carry risk management or warning labels. These are described in Table 1.
Table 1: Summary of other biobased products which may carry risk management or warning labels
|
Material |
Application in food (FCM) |
Allergy hazard(s) |
Business operator |
Other comments |
|
Wheat |
Drinking straws |
Allergy to wheat protein and/or gluten |
The wheat straws company |
As the straws may be sold loosely, the FSA recommended that the business provides adequate labelling and/or information provided to the end user. |
|
Sodium alginate (derived from seaweed) and three other ingredients (additives) |
Food packaging wraps |
Allergy to shellfish protein and/or seaweed |
Algaewrap |
The company said that they no longer planned to market the material as edible, and had documentation stating that it did not contain allergenic proteins. |
|
Film made from shellfish waste including chitosan |
Secondary FCM packaging (to provide indirect contact and rigidity) |
Allergy to shellfish protein |
Shellworks and a UK start-up business developing chitosan products for food applications which are not yet on the market (UK Start-up) |
Shellworks: further advice given from the FSA was that labelling needed to be clear and further considerations needed to be taken into account when anything is served loosely (individually), should any item likely fall into that category.
UK Start Up: the FSA suggested that all allergenic substances should be removed from the final material to mitigate the risk as much as possible. |
16. Regarding Table 1, and as per article 15(1)b of retained EU Regulation 1935/2004, materials and articles which are not yet in contact with food when placed on the market should be accompanied by, if necessary, special instructions for safe and appropriate use. However, any wording used by the business operators on the health warning or risk management is not known. The FSA FCM team is not aware of any warning labels currently in use for these products; none have been drawn to their attention, and they have not come across such labels in similar products. Whilst labels may alert vulnerable groups, it seems unlikely such a product would be marketable if such a danger was recognised to the point that a label was required.
Biological sources of chitosan
17. Fera Science Ltd (Sand Hutton, York, UK) currently has a PhD research project on chitosan films. The project involves extraction, production, and characterisation of chitosan from farmed black soldier fly. The characterisation of the chitosan involves measuring the degree of acetylation, molecular weight distribution, and assessing its antimicrobial activity against different foodborne pathogens. These films are intended to be used in shelf-life extension applications, such as edible coatings to foods susceptible to spoilage and pathogenic microorganisms. However, this work does not include safety or migration testing.
18. The most investigated species for fungal chitosan production include Aspergillus niger (Ascomycota), Lentinus edodes (Basidiomycota), Absidia coerulea, and Absidia glauca (Zygomycota), Rhizophus oryzae, and Mucor rouxii (Mucoromycota). However, production of fungal chitosan has not yet been scaled up to an industrial level. (Hahn et al. 2020). Presently, three notices appear on the US FDA website for fungal-derived chitin and chitosan in food applications, where the fungal species used are Agaricus bisporus and A. niger. For example, one notice is for A. niger-derived chitosan, used as a “direct food ingredient in alcoholic beverage production at levels between 10 and 500 grams per hectoliter”, by KitoZyme (GRN no. 397); the US FDA had no questions on this notice, and it was closed in 2011. The UK FSA has not had any applications for the use of chitosan as a FCM.
Exposure assessment
19. The COT requested an estimation of total exposures to allergenic proteins in BBFCMs in contact with different foods. In respect of this, some preliminary exposure calculations are presented in annex B. Annex B is reserved as it contains confidential information from a company developing chitosan products for food applications which are not yet on the UK market.
20. The following position paper (annex A) summarises discussions that have
taken place so far at COT and future work. Since further information, particularly on exposure assessment, is anticipated in the short to medium term, a position paper rather than a statement has been prepared with a view to future revision.
Questions for the Committee
1) Do Members have any comments on the new information provided?
2) Which of the new information should be included in the position paper (if any)?
3) Does the position paper (annex A) outline and summarise the discussions thus far on allergenicity of chitin and chitosan based BBFCMs?
4) Does the Committee have any comments on the structure or content of the draft position paper?
5) Do Members have any comments on the draft exposure calculations in annex B?
Secretariat
August 2022
References
Baumann H. & Tillman A.M. (2004) The Hitch Hiker’s Guide to LCA. An Orientation in Life Cycle Assessment Methodology and Application. Studentlitteratur, Lund, Sweden.
Codex (2005) General standard for fruit juices and nectars (Codex Stan 247-2005, p.6). Available at: GENERAL STANDARD FOR FRUIT JUICES AND NECTARS (fao.org)
Ghosh T. & Katiyar V. (2020). Ch. 16 Life Cycle Assessment of Chitosan. In book: Advances in Sustainable Polymers. Springer. Eds: Katiyar V., Kumar A., Mulchandani N. (pp.380-381)
Hahn T., Tafi E., Paul A., et al. (2020) Current state of chitin purification and
chitosan production from insects. Journal of Chemical Technology and Biotechnology 95: 2775-2795
Kim W.H., Lee S.K., Lee H.C., et al. (1982) Shell-grinder’s asthma. Yonsei Medical Journal 23: 123-130
Leceta I., Guerrero P., Cabezudo S., et al. (2013) Environmental assessment of chitosan-based films. Journal of Cleaner Production 41: 312-318
Madival S., Auras R., Singh S.P., et al. (2009) Assessment of the environmental profile of PLA, PET and PS clamshell containers using LCA methodology. J Clean Prod 17: 1183-1194
Nagano T., Janao K., Sugai T., et al. (1984) Allergic contact urticaria caused by raw prawns and shrimps: Three cases. Journal of Allergy and Clinical Immunology 74: 489-493
Nguyen M.X.H. (2012) Characterization of allergenic and antimicrobial properties of chitin and chitosan and formulation of chitosan-based edible film for instant food casing. Melbourne, Australia: Royal Melbourne Institute of Technology (RMIT) University, PhD thesis. Available at: 15625643.pdf (core.ac.uk)
Suwanmanee S. & Lertworasirikul S. (2015) Environmental impact assessment of coating fresh-cut papaya Cv. Holland with chitosan. In: Proceedings of the 35th the IIER international conference, Bangkok, Thailand. ISBN 978-93085465-89-5 Available at: Microsoft Word - II-STMBGK-02095-02 (worldresearchlibrary.org)
Annex A to TOX/2022/45
Position paper on chitosan in bio-based food contact materials
Background
1. The use of fossil-based plastics has been associated with adverse environmental impacts. Consequently, there is greater interest in reducing the amount of conventional plastic used for packaging, and recent years have seen a major global increase in the development and use of bio-based food contact materials (BBFCMs). Bio-based materials are defined as being derived, directly or indirectly, from a renewable source of living matter (Bradley, 2010).
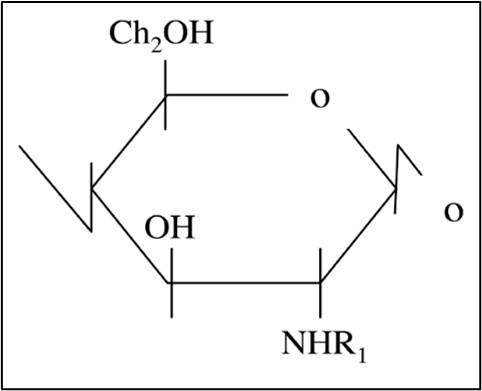
2. Some BBFCMs under development contain chitosan, which is a biodegradable polysaccharide derived from chitin (Figure 1). Chitosan has antimicrobial and antioxidant properties which make it ideal for extending the shelf-life of packaged foods (Vasile, 2018).
3. Chitin is a high molecular weight β(1,4)-linked homopolymer of N-acetylglucosamine (see Figure 1). In situ, chitin is linked to other structural components, such as protein and glucan, to form a protein-chitin matrix (Romano et al., 2007). Chitin is converted to chitosan by removing the acetyl groups (COCH3).
Figure 1: Chemical structures of chitin (R1 = COCH3) and chitosan (R1 = H).
4. Presently, the main commercial source of chitosan is from chitin obtained from waste streams of the marine fishery industry, i.e. crustacean shells. However, the recent increased global demand for chitosan has drawn attention to other possible sources: fungi and insects.
5. Production of chitosan from chitin involves deproteination and subsequently deacetylation. However, since the level of deproteination reported in literature studies is <100 % (and any residual protein in chitosan might contain allergenic proteins), there is a concern regarding the potential allergenicity of chitosan when used in FCM.
Tropomyosin
6. Tropomyosin is a protein present in all species of vertebrates and invertebrates. However, only the tropomyosin found in invertebrates such as crustaceans, arachnids, insects, and molluscs is associated with allergic reactions in humans (Reese et al., 1999).
7. Tropomyosin is a heat-stable allergen (Daul et al., 1994). It is also an “acidic” protein with an isoelectric point (pI) value of 4.5 (Reese et al., 1999). Due to these characteristics, tropomyosin can be present in processed foods (Reese et al., 1999). However, in their review of shellfish allergy, Woo & Bahna (2011) note that tropomyosin’s “allergenicity may change by certain processing methods”, such as boiling and ultrasonication.
8. Nguyen (2012) used immunoblotting techniques to demonstrate the presence of tropomyosin in samples of shellfish-derived chitin and chitosan, where antibodies were able to interact with tropomyosin (concentrations of tropomyosin in these samples were not reported). Subsequently, Nguyen (2012) noted that “special care should be taken when using chitin and chitosan in food or medical preparations. Warning statements should state clearly the presence of tropomyosin in products derived from chitin or chitosan, especially when the consumers are sensitised to crustaceans”.
Chitin & chitosan-based BBFCMs in development
9. Chitosan is used in food packaging in the form of flexible films or coatings. A “film” is preformed separately and wrapped onto a food surface. These films are usually prepared by using a solvent casting method, in which chitosan is dissolved in suitable solvents (in most cases, slightly acidified water) and then poured onto a flat surface to allow the solvent to evaporate (Kim et al., 2006). On the other hand, a “coating” is a thin layer formed directly onto the food’s surface. Direct application of chitosan formulations onto food surfaces can be attained by spraying or dipping (Tharanathan, 2003).
10. In literature studies, chitosan-based films may be described as edible or inedible, whereas chitosan coatings are almost always described as edible since they form a layer directly on the top surface of the food (Priyadarshi & Rhim, 2020). Another difference is that chitosan films are >30 µm in thickness, whilst coatings are <30 µm in thickness (Van den Broek et al., 2015). However, with the advancement of nanotechnology, nano-coatings are being explored, which consist of nanoscale layers (<100 nm) built-up onto food surfaces (Vasile, 2018). In their review of “nanoedible films” for food packaging, Jeevahan & Chandrasekaran (2019) noted that production of edible films and coatings is still largely at the laboratory level and is not yet expanded to industrial level due to their high cost of production.
11. It is suggested that chitosan-based films could appear in vacuum-packaged processed meat (Ouattara et al., 2000), cheese (Fajardo et al., 2010), and other foods such as vegetables, fruits, grains, and fish (Sinha et al., 2012).
12. Modifying chitosan by the addition of a metal has been shown to enhance its antimicrobial activity compared to native chitosan (Du et al., 2009). Subsequently, some chitosan-based BBFCMs in development are nanoengineered to contain metal ions, such as copper (Yin et al., 2018).
13. Some BBFCMs in development contain chitin, in the form of nanofibers (Ifuku & Saimoto, 2012) or “nano-whiskers” (Zeng et al., 2012). Incorporation of chitin nano-whiskers into starch-based films has been shown to improve the film’s mechanical and barrier properties (Qin et al., 2016). Regarding chitin nano-whiskers, migration studies are scarce. This is due to the difficulties in characterising nanoparticles in composites generally, and the lack of methods for qualitative and quantitative analysis (Han et al., 2011).
Market uses of chitosan
14. Chitosan has applications in tissue engineering and biomedicine due to its low cost, biocompatibility, lack of toxicity, and biodegradability (Madhumathi et al., 2009; Konovalova et al., 2017).
15. Chitosan is widely used in as a food additive and functional ingredient in foods sold in Italy, Finland, Korea and Japan (Peter, 1997; Singla & Chawla, 2001). Both chitin and chitosan are approved food additives in Japan (JFCRF, 2011). Furthermore, chitosan is listed as a processing aid in the Codex General Standard for Fruit Juices and Nectars (Codex, 2005). The Norwegian company “Norwegian Chitosan AS” trades chitosan (Kitoflokk™ and Norlife) for several applications, including food and beverages (Ferreira et al. 2016).
16. Chitosan and chitin have not been officially classified as GRAS (generally recognised as safe) by the US Food and Drug Administration (US FDA). Rather, some biotechnology companies have notified the US FDA of their view that the use of chitosan and chitin in specific food applications is GRAS. For example, KitoZyme views the use of chitosan (derived from Aspergillus niger) in alcoholic beverage production (with chitosan being removed from the beverages post-treatment, using physical separation processes) as GRAS. In their correspondence to KitoZyme, the US FDA (2011) wrote: “based on the information provided by KitoZyme, as well as other information available to FDA, the agency has no questions at this time regarding KitoZyme's conclusion that chitosan from A. niger is GRAS under the intended conditions of use. The agency has not, however, made its own determination regarding the GRAS status of the subject use of chitosan”.
17. Shellfish-derived chitosan is sold online as a dietary supplement, where manufacturer-recommended daily consumption of chitosan is, for example, 2.4 g and 3 g. The idea is that chitosan may support weight loss and lower cholesterol by eliminating fat and cholesterol from the body instead of allowing the body to absorb them (Moraru et al. 2018).
18. Chitosan is considered to be hemostatic due to its cationic nature (NTP, 2017), which supports its use in wound dressings. Wound dressings manufactured from shellfish-derived chitosan were introduced in 2005 for US soldiers, and in 2008 the US FDA approved the HemCon bandage for use as a dressing for local management of bleeding wounds (US FDA, 2008).
ADME
19. Results from Chae et al. (2005) indicate that absorption of chitosan from the gastrointestinal tract following oral exposure in rats is inversely related to its molecular weight: oral gavage administration of chitosan with molecular weights of 3.8, 7.5, 13, 22, or 230 kDa resulted in maximum plasma concentrations of 20, 9, 6, 4, or <0.5 μg chitosan/mL, respectively.
20. Several mammalian chitinases have been identified in humans which can bind and degrade chitin (Boot et al., 2001). Furthermore, Lactococcus lactis and Lactobacillus plantarum have chitinolytic and/or chitin-binding proteins (Sánchez et al., 2011). These bacteria are an integral part of normal gut flora, fermented foods, and probiotic-fortified foods (Kim et al., 2013; Todorov et al., 2012).
21. Degradation of chitosan in vertebrates is thought to occur predominantly by lysozymes and bacterial enzymes in the colon (Kean & Thanou, 2010).
22. The depolymerised products of chitin or chitosan are called chitooligosaccharides (COS), which have a molecular weight of approximately 10 kDa or less (Xia et al., 2010). COS are water-soluble (Qin et al., 2006), and have antioxidative, anti-inflammatory, and antibacterial effects (Huang et al., 2016). However, COS have been observed to irritate intestinal epithelial mucosal tissues, stimulating them to produce mucin (Deters et al., 2008). Following depolymerisation, both chitin and chitosan particles are readily phagocytosed (Bueter et al., 2011).
Allergen reference doses
23. The most widely accepted allergen reference doses for crustacean-derived protein are an ED01 (where <1 % of the allergic population may be expected to react) at 26.2 mg (95 % confidence interval of 2.7-166 mg) of shrimp protein, and ED05 at 280 mg (95 % confidence interval of 69.3-880 mg) (Remington et al., 2020). These reference values are derived from human food challenge data, and represent acute intake levels of crustacean-derived protein that are predicted to provoke an objective reaction in no more than 1 and 5 % (respectively) of at-risk individuals, who show a minimal allergic response upon challenge. An allergenic reference dose for tropomyosin alone was not identified in the literature (other allergenic proteins, in addition to tropomyosin, are present in shellfish protein such as arginine kinase).
24. In EFSA’s 2014 evaluation of allergenic foods and food ingredients for labelling purposes, EFSA noted that “studies reporting on the prevalence of allergy to crustaceans in the general (unselected) European population are scarce. In the few studies available, the prevalence of self-reported crustacean-related adverse reactions to food in children ranged from 0.1 % and 0.3 % in Greece (Zannikos et al., 2008) and the UK (Pereira et al., 2005) to 5.5 % in France (Touraine et al., 2002). Figures reported from the Netherlands (Brugman et al., 1998), Sweden and Iceland (Kristjansson et al., 1999) were within that range (0.7–1.5 %). Prevalence of self-reported allergy to shrimps was 0.5 % in 2- to 14-year-old Finch [sic] children (Rancé et al., 2005). In adults, estimated sensitisation rates to crab in Germany (Schafer et al., 2001) based on positive SPTs were similar to those reported in Hungary (Bakos et al., 2006) based on specific IgE testing (1.9 % and 1.8 %, respectively). Prevalence rates of allergy to crustaceans based on clinical history and positive SPT in the German general population were much lower (0.2 %) (Zuberbier et al., 2004). Only one study conducted in Denmark reported challenge proven prevalence data for shrimp allergy, which ranged from zero in subjects < 22 years to 0.3 % in subjects > 22 years (Osterballe et al., 2005)”.
25. Pereira et al. (2005) investigated the rates of food hypersensitivity (FHS) in UK teenagers against a panel of allergens including shellfish. These teenagers (11 and 15 year-olds) were resident on the Isle of Wight at the time of the study, and completed a questionnaire with their parents on adverse reactions to foods. According to table 1 of this publication, 2 eleven year-olds (0.3 %; n = 699), and 5 fifteen year-olds (0.8 %; n = 649) reported an adverse event from consumption of shellfish.
26. Young et al. (1994) conducted a population study to identify the prevalence of reactions to eight foods commonly perceived to cause sensitivity in the UK, including “fish/shellfish (as prawn)”. Following a survey distributed nationwide to UK households, 8,328 individuals completed the survey, 236 (2.8 %) of which reported a reaction to “fish/shellfish”.
Case reports of reactions to chitosan
27. Kato et al. (2005) reported a case of immediate-type allergy from use of a health food containing chitosan, where “The patient was a 47-year-old female person who developed systemic urticaria and difficulty in breathing after oral ingestion of chitosan. Since skin tests (prick test and scratch patch test) were positive, the test was done using another commercial chitosan, and was positive. The patient was diagnosed as having chitosan-induced immediately-type allergy, and was instructed to avoid ingestion of chitosan. The patient developed no symptoms thereafter”. The study authors concluded that chitosan may have functioned as a food allergen because of its molecular weight and general properties.
28. Two case reports were identified relating to hypersensitivity to some healthcare products containing chitosan (Cleenewerck et al., 1994; Pereira et al., 1998). The biological source of the chitosan is not stated in these publications.
Reactions from entomophagy
29. Reports on adverse reactions from insect consumption (entomophagy) are scarce and only two population studies (described below) were identified in the literature which report on the prevalence of food allergy to insects. In these two studies, clinical measurements of allergy do not seem to have been verified, which is a limitation of the data.
30. Taylor & Wang (2018) investigated the prevalence of allergic reactions caused by consuming edible insects. The investigation was conducted in the North Eastern (or the Isan region) of Thailand, in an area where entomophagy is common. Information concerning entomophagy and allergic reactions were gathered from multiple sources in four locations: Nongki, Nang Rong, Nong Bun Mak, and Nakhon Ratchasima. The survey included questions about eating habits in relation to insects, other known food allergies, and presented a list of symptoms the participants may have experienced. The prevalence of allergic reactions caused by consuming edible insects was much higher than expected across the 2,500 respondents. In the Isan region, approximately 14.7 % of people experienced a single symptom indicative of an edible-insect allergy, and 7.4 % of people experienced multiple symptoms “indicative” of an edible-insect allergy. Furthermore, approximately 46.2 % of people that already suffer from a known food-based allergy also experienced symptoms indicative of an allergic reaction after insect consumption. According to the study authors, “the most common symptoms appear to be gastrointestinal (diarrhoea and vomiting)”. The study authors concluded that “the allergy aspect of entomophagy is a serious issue and has the potential to adversely affect the future of entomophagy, especially in introducing the concept to western cultures”.
31. Barennes et al. (2015) assessed the prevalence of food allergy to insects amongst insect-eaters. In this survey, eight teams (which included medical physicians) collected data to address socioeconomic characteristics of the consumers, types of insects consumed, frequency of consumption and reports of side effects. This study was conducted in Laos, and included 1,059 subjects that had previously eaten insects, 81 of whom (7.6 %) reported “allergy problems after eating insects”. Of these 81 subjects, 38 reported that allergy problems were “mostly with grasshoppers or stink bugs”. None of the subjects reported severe anaphylaxis. In this survey, it was not possible to know how much the consumption of edible insects represents the daily diet of the population, or provide detail on the way insects were harvested. It does not mention any clinical confirmation of the allergenic symptoms.
UK incidents
32. The FSA has received a number of queries about the presence of chitosan in food packaging materials and chitosan-based drinking straws, but no incidents have formally been raised within the FSA. However, there was one report of a potential reaction to the use of a chitosan-based straw in a pub which was reported to a local authority. The local authority carried out an investigation with the supplier of the chitosan-based straws. However, in this case, it difficult to rule out cross-contamination from the meal that the individual had also consumed on the premises. The individual who suffered the allergic reaction did have a seafood allergy but did not disclose this to the pub. That is the only incident that has been reported to FCM team. It was and remains unsubstantiated.
UK legislative position
33. In retained European legislation, all materials and articles intended for contact with food must meet the requirements of the Framework Regulation (EC) No. 1935/2004. The principle underlying this Regulation is detailed in Article 3 which states: “materials and articles, including active and intelligent materials and articles, shall be manufactured in compliance with good manufacturing practice so that, under normal or foreseeable conditions of use, they do not transfer their constituents to food in quantities which could: a) endanger human health; b) bring about an unacceptable change in the composition of the food; c) bring about a deterioration in the organoleptic characteristics thereof.”
34. With regards to necessary labelling (and potential exposure to allergens) Article 15 of retained Regulation (EC) No 1935/2004 states that ‘special instructions (are) to be observed for safe and appropriate use’. This labelling information may need to be provided on the packaging, or as a standalone warning should the item be sold loosely. If the item was marketed as edible, other labelling requirements come into play to comply with food law and the Materials and Articles in Contact with Food Regulations 2012 as amended.
35. Whilst there are no specific migration limits for BBFCMs, industry can refer to legislation that may be pertinent (the same holds true for other materials lacking specific legislation). Furthermore, the Plastics Regulation stipulates a generic migration limit of 10 mg per square decimetre of surface area of material (10 mg/dm2) which is applicable under these circumstances (this is equivalent to 60 mg of total constituents released per kg of food simulant). The applicability of FCM legislation depends on the BBFCM’s intended use and how it is marketed. If the BBFCM is intended purely for containment purposes and is inedible, it is not food and comes under FCM legislation.
36. The EU considers that an edible film is a special active part of the food and, seen from a legal point of view, it is to be regarded as a foodstuff, along with the food packed in the film, having to fulfil the general requirements for food (Fabec et al., 2000). Subsequently, the presence of a known allergen in an edible film or coating on a food must be clearly stated in the label (Campos et al., 2011). Due to hygienic reasons, it is anticipated that food products in edible films need to have an outer package, otherwise the film should not be eaten (Fabec et al., 2000).
Evaluations of crustacean chitosan
2011 Evaluation by EFSA (EFSA NDA Panel)
37. In 2011, when reviewing a proposed health claim for a food supplement containing crustacean-derived chitosan, the EFSA NDA Panel concluded, that “a cause and effect relationship has been established between the consumption of chitosan and maintenance of normal blood LDL-cholesterol concentrations”, and “considers that in order to obtain this effect in adults, 3 g of chitosan should be consumed daily” (EFSA, 2011). The Panel stated that their opinion does not constitute, and cannot be construed as, a positive assessment of its safety.
Literature studies
38. Studies designed to evaluate the effectiveness of shellfish-derived chitosan as an oral weight-loss supplement over 12 days suggest that it is well tolerated in men and women at 4.5 g chitosan per day (Gades & Stern 2003, 2005). Data collection sheets for the volunteers did not appear to have a space for recording any adverse effects, but one of the 15 male participants reported “vomiting after a meal during the supplement period” (Gades & Stern 2003). Additionally, in a study involving 65 men and women, consumption of chitosan tablets (6.75 grams of chitosan daily for eight weeks), was “found to be safe”, though common transient gastrointestinal symptoms were reported (loose faeces, constipation, abdominal pain, repeated flatulence, abdominal bloating, and abdominal rumbling) (Tapola et al., 2008).
39. In 2011, Waibel et al. investigated the safety of “HemCon®” bandages in patients who reported shellfish allergy. Initial assessment included a detailed history, IgE SPT, and serum testing to shellfish allergens. Participants who demonstrated specific shellfish IgE underwent a bandage challenge. It was reported that of the nineteen participants who were enrolled, 10 completed the study. Seven (70 %) were male and the average age was “44.8 + [sic] 10 years”. Nine (90 %) reported a shrimp allergy history and five (50 %) reported multiple shellfish allergies. All participants completing the study had positive SPT and serum IgE testing to at least one shellfish; eight (80 %) had shrimp positive SPT and ten (100 %) demonstrated shrimp-specific IgE. No participant had a positive SPT to chitosan powder or experienced an adverse reaction during bandage challenges. No protein bands were visualised during gel electrophoresis analysis of chitosan powder. The study authors concluded that all participants tolerated the HemCon bandage without reaction.
Evaluations of fungal chitin
2010 Evaluation by EFSA (EFSA NDA Panel)
40. In 2010, the EFSA panel on Dietetic Products, Nutrition and Allergies (NDA) assessed the safety of chitin-glucan as a novel food ingredient (EFSA, 2010). This chitin-glucan was derived from A. niger through a fermentation process, and therefore did not contain shellfish protein. The product assessed by EFSA was called “KiOnutrime-CG™”, composed of >90 % chitin-glucan (a structure that combines chitin and beta (1,3) glucan) and ≤ 6 % protein, and was intended to provide a daily intake of 2 - 5 grams of chitin-glucan. The Panel reviewed a report showing no observed adverse effects at the highest dose administered (about 6.6 g/kg bw) in a 13-week rat study (TNO, 2009). Because this dose is approximately 80-fold higher than the maximum intended level of intake for humans on a g/kg bw basis, the Panel concluded that KiOnutrime-CG™ was safe as a food ingredient at the proposed conditions of use and at the proposed intake levels. The Panel assessed the risk of allergenicity on the basis of some allergenic enzymes that are synthesised by A. niger such as beta-xylosidase. The Panel concluded that “an allergenic risk cannot be ruled out, but is expected not to be higher than the consumption of other A. niger derived products”. The Panel also noted that since A. niger is commonly detected in various foods such as fruits and vegetables, it is therefore expected to occur in the diet of most individuals.
2012 Evaluation by FSANZ
41. In 2012, Food Standards Australia New Zealand (FSANZ) approved an application for the use of A. niger-derived chitosan as a processing aid for production of some beverages. In their risk assessment, FSANZ noted that animal toxicity studies on chitosan preparations of various molecular weights and degrees of acetylation did not show any treatment-related adverse effects following oral administration at high doses. Furthermore, “a published review of human data from 13 clinical trials of up to 6 months duration found no adverse effects associated with oral chitosan (average daily dose 3.5 g) as a weight loss supplement. In view of the absence of adverse effects at high chitosan doses, a group Acceptable Daily Intake (ADI) “not specified” was established for chitosan derived from fungi. Information was provided indicating negligible levels of fungal chitosan in wine following processing. Negligible levels would also be expected in beer and cider, while no residual fungal chitosan would be expected in alcoholic products derived from distillation”. The overall conclusion was that the “use of fungal chitosan as a processing aid for the production of wine, beer, cider, spirits and food grade ethanol is technologically justified and raises no public health and safety issues for consumers” (FSANZ, 2012).
National Aspergillosis Centre (NAC)
42. The NAC is a service commissioned by the UK National Health Service to diagnose and manage chronic aspergillosis. The NAC notes that: “several of the papers already described mention that Aspergillus can cross react with other fungi including Cladosporium, Alternaria and Fusarium. This suggests that Aspergillus could very rarely cause food allergies such as we have described for the other three fungi, but we have no evidence that they actually do. Much more work is needed in this area of allergy research before we can make firm conclusions”. Additionally, the NAC noted that: “eating the fungal-based food Quorn (made from Fusarium) triggered an allergic reaction based on cross reactivity of a Quorn allergen to an allergen in airborne fungi that the patient was allergic to (Hoff et al., 2003) – there are anecdotal reports of more cases of this type of allergy but only very few” (UK NAC, 2022).
Literature studies
43. Seaton & Wales (1994) conducted an 8-year follow-up study on clinical reactions to A. niger in a biotechnology plant producing citric acid by fermentation of molasses with A. niger. The authors concluded that A. niger was a weak antigen, and that simple hygiene measures were needed to protect the workforce.
44. Mycoprotein is a food produced for human consumption by fermentation of Fusarium graminearum on a glucose substrate. Known as ‘Quorn’, it is widely available in most leading UK retailers where it has been on sale since 1985. To investigate whether mycoprotein would cause allergic responses either in exposed production workers, or in those ingesting ‘Quorn’, immunological studies were conducted by Tee et al. (1993). Mycoprotein production workers were screened for allergy using the radioallergosorbent test (RAST) over a 2 year period. Two of 10 patients referred to hospital following vomiting and diarrhoea after ingestion of mycoprotein had a mycoprotein skin-prick test (SPT) response ≥2 mm, but none had a significantly raised RAST. Specific IgE antibody to mycoprotein was not significantly raised in any complainant. The study authors concluded that “the possibility can not be excluded of participation of fungal polysaccharide allergens, which RAST testing would probably not detect, or of non-lgE associated mechanisms. Intolerance to ingested Quorn reported by a small number of consumers may be due mainly to an idiosyncratic response”.
Evaluations of insect chitin
2020 Evaluation by EFSA (EFSA NDA Panel)
45. In 2020, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) published their opinion on the safety of dried yellow mealworm (Tenebrio molitor larva) as a NF (novel food) (EFSA, 2020). The reported average chitin content of the NF in powder form was reported to be 6.42 ± 0.28 g /100 g across 5 batches (the NF was not reported to contain chitosan).
46. The NDA Panel noted that “yellow mealworms are consumed as part of the customary diet or for medicinal purposes in some non-EU countries worldwide. Their consumption by humans has been reported in Thailand (Hanboonsong et al., 2013), China (Feng et al., 2018) and Mexico (Ramos-Elorduy, 1997, 2009; Ramos-Elorduy and Moreno, 2004). Yellow mealworms are among the insect species permitted to be consumed as food in Korea by the Korean Food and Drug Administration (KFDA) (Kim et al., 2017). Additionally, in Australia and New Zealand yellow mealworms are considered as non-traditional, not novel foodstuff (FSANZ, 2020). Since 1 May 2017, T. molitor larva is among the insect species that can be legally introduced in the Swiss market as food (whole, chopped or ground)” (EFSA, 2020). Because of this history of use, and the absence of adverse effects described in the literature, the Panel concluded that “the NF is safe under the proposed uses and use levels”. The proposed use was as an ingredient in several food products, such as pasta-based dishes, and biscuits), for all population groups.
47. The NDA Panel also discussed the work of Broekman et al. (2017) (which demonstrates the possibility of de novo human sensitisation to allergens in mealworm, which can result in food allergy), and of Broekman et al. (2015) (which demonstrated that thermal processing did not lower the allergic potential of mealworm allergens) (EFSA, 2020). The Panel also noted that “the applicant provided the study of Velasquez (2015) who investigated the allergenic potential of yellow mealworm larvae using extracts of the NF, and concluded that subjects allergic to arthropods and more specifically to crustaceans, should not consume the NF due to the risk of cross-reactivity” (nb. this study is a Master’s thesis which is written in French). Subsequently, the NDA Panel considered that “the consumption of the NF may induce primary sensitisation and allergic reactions to yellow mealworm proteins and may cause allergic reactions in subjects with allergy to crustaceans and dust mites. Additionally, allergens from the feed may end up in the NF”. Furthermore, the Panel recommended that research should be undertaken on the allergenicity of yellow mealworm, including cross-reactivity to other allergens.
Literature studies
48. Broekman et al. (2016) included 15 patients with shrimp allergy (based on specialist opinion and diagnostic testing) in a double-blind, placebo-controlled food challenge (DBPCFC) trial, and found that 13 of these patients also had mealworm allergy. The study authors noted that “when comparing the mealworm challenge outcome of 4 patients who also had a shrimp challenge, eliciting doses (ED5 and ED10) as well as severity were in the same range”, which “indicate(s) that mealworm is at least as allergenic as shrimp”, though “more mealworm challenge data are needed to confirm this initial analysis”. Subsequently, Garino et al. (2020) used the data from Broekman et al. (2016) to predict values for the ED05, ED10, and ED20, indicating where 5 %, 10 % and 20 % of the shrimp allergic population are predicted to react to mealworm proteins. The values for the ED05 were 63, 128, and 147 mg of T. molitor protein, estimated using the Weibull, log-logistic, and log-probit distribution models, respectively.
FSA response
49. A first draft statement on the potential allergenicity of chitosan in FCMs was presented to the COT on 7th September, 2021. Furthermore, the FSA is corresponding with a biotechnology company which is developing food packaging materials comprised of chitosan, as this provides a useful addition to the current state of knowledge for these materials.
COT consideration
50. The COT considered that the risk of allergenicity from chitin- or chitosan-based BBFCMs on the basis of the potential presence of allergenic proteins appears to be low. However, to confirm this, more information is needed, in particular additional data characterising the protein content in chitosan and the final BBFCMs (against chemical and enzymatic methods of deproteination) would be useful, together with data on migration from, and consumption of, BBFCMs. Information on the total amount of residual protein (expressed as mg/g BBFCM) would be helpful for estimating health risks.
51. The COT considered that available clinical ingestion data indicate that the immunological properties of chitin and chitosan are of low concern in the context of BBCFMs (Gades & Stern 2003, 2005; Tapola et al., 2008). Chitin is well tolerated in supplements at higher exposures than would be expected from the use of BBFCMs. However, some adverse effects were associated with high intakes of the raw materials in clinical studies, which were typically mild symptoms of gastrointestinal tract distress such as diarrhoea, bloating, or vomiting. The indications are that this is a relatively non-specific inflammatory reaction. It was agreed that these adverse effects were not of concern for BBFCMs as the processing is likely to produce a more inert final material. Furthermore, the phagocytosis of small fragments of chitin or chitosan appeared to be the same as that of similar-sized particles in general.
52. Regarding the reported case of immediate-type allergy for chitosan-containing health food (Kato et al., 2005), the COT agreed that the limited information provided in this case report did not suggest any additional concerns. It was considered that this reported case of immediate-type allergy is most likely due to residuals from the shellfish source from which the chitosan supplement was derived.
53. Regarding the two case reports of hypersensitivity to some healthcare products containing chitosan (Cleenewerck et al., 1994; Pereira et al., 1998), the COT agreed that the type of hypersensitivity described in these two cases very rarely, if ever, occurs in the context of food ingestion.
54. A submission by Primex to the US FDA in 2012 (GRAS Notice No. 443) contains a dossier which includes some approaches to protein measurement and analytical data for the ED01 and corresponding analysis . This dossier was considered by the COT in discussion paper TOX/2021/03, where the COT noted that the chitosan used in this submission appeared to be highly controlled in terms of its production; and whilst its specification may be unlike that of other chitosan products, it nevertheless provides a standard to be achieved and possibly put forward.
55. The COT considered that the ED01 is an adequate protection goal, given the potential for increased human exposure to the allergen if it were to be present in food packaging. It was agreed that the choice of benchmark (e.g. ED01) is a risk management decision or benchmark. Due to the large amount of data required for dose distribution modelling, accurate estimates below ED01 are not feasible. As noted above, the ED01 is the amount of shellfish protein consumed that causes a reaction in 1 % of the allergic population. If 2.8 % of the population are estimated to be sensitive to shellfish protein, then in theory, the probability of a reaction in a randomly-chosen UK individual exposed to the ED01 would be 1 % of 2.8 %, i.e. 0.028 %. Despite this low percentage, widespread usage may affect a significant number of people. Therefore, appropriate risk management measures are important, such as labelling to declare allergenic source(s), and consumer awareness unless exemptions are obtained.
56. The COT considered that in order to assess whether FCM posed a negligible health risk in practice (if consumption was below the ED01), it would be necessary to understand the effects of processing on the levels of allergens in the final FCM, which may then migrate into food (as is the case for other allergens).
Next steps
57. Measurements of the amount of allergenic protein in BBFCMs have not been identified in the scientific literature at present. No public usage or consumption data for chitin or chitosan based BBFCMs were identified in the literature or the National Diet and Nutrition Survey (NDNS) database.
58. When such information becomes available, it could be used to provide an indication or estimation of users’ exposures to any allergenic proteins in chitin or chitosan-based BBFCMs. These exposures could be compared against the relevant ED01 values for assessment of health risk.
COT draft position paper
September 2022
List of Abbreviations
|
BBFCM |
bio-based food contact material |
|
Bw |
bodyweight |
|
COS |
chitooligosaccharide |
|
ED |
eliciting dose |
|
FCM |
food contact material |
|
GRAS |
generally recognised as safe |
|
KDa |
kilodaltons |
|
NDNS |
national diet and nutrition survey |
|
OML |
overall migration limit |
|
PLA |
poly(lactic) acid |
|
Ppb |
parts per billion |
|
SML |
specific migration limit |
|
SPT |
skin prick test |
References
Barennes H., Phimmasane M., & Rajaonarivo C. (2015) Insect consumption to address undernutrition, a national survey on the prevalence of insect consumption among adults and vendors in Laos. PLoS ONE 10(8): e0136458.
Bradley E.L. (2010). FSA Project A03070: Biobased materials used in food contact applications: an assessment of the migration potential. Available at:
Broekman H.C., Knulst A.C., den Hartog Jager S., et al. (2015) Effect of thermal processing on mealworm allergenicity. Molecular Nutrition and Food Research 59: 1855-1864.
Broekman H., Verhoeckx K.C., den Hartog Jager C.F., et al. (2016) Majority of shrimp-allergic patients are allergic to mealworm. J. Allergy Clin. Immunol. 137: 1261-1263.
Broekman H.C., Knulst A.C., den Hartog Jager C.F., et al. (2017) Primary respiratory and food allergy to mealworm. Journal of Allergy and Clinical Immunology 140: 600-603.
Bueter C.L., Lee C.K., Rathinam V.A., et al. (2011) Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J Biol Chem 286: 35447-35455.
Boot R.G., Blommaart E.F., Swart E., et al. (2001). Identification of a novel acid mammalian chitinase distinct from chitotriosidase. Journal of Biological Chemistry 276: 6770-6778.
Campos C.A., Gerschenson L.N., & Flores S.K. (2011). Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 4: 849-875.
Chae S.Y., Jang M.K., & Nah J.W. (2005) Influence of molecular weight on oral absorption of water soluble chitosans. J Control Release. 102(2): 383-394.
Cleenewerck M.B., Martin P., & Laurent D. (1994). Allergic contact dermatitis due to a moisturizing body cream with chitin. Contact Dermatitis 31(3): 196-197.
Daul C.B., Slattery M., Reese G., et al. (1994) Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int. Arch. Allergy Immunol. 105: 49-55.
Deters A., Petereit F., Schmidgall J., et al., (2008) N-acetyl-d-glucosamineoligosaccharides induce mucin secretion from colonic tissue and inducedifferentiation of human keratinocytes, J. Pharm. Pharmacol. 60: 197-204.
Du W.L., Niu S.S., Xu Y.L., et al. (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym. 75(3): 385-389.
EFSA (2010) Scientific Opinion on the safety of ‘Chitin-glucan’ as a Novel Food ingredient. EFSA Journal 8(7): 1687 Available at: Scientific Opinion on the safety of ‘Chitin‐glucan’ as a Novel Food ingredient - - 2010 - EFSA Journal - Wiley Online Library.
EFSA (2011) Scientific Opinion on the substantiation of health claims related to chitosan and reduction in body weight (ID 679, 1499), maintenance of normal blood LDL-cholesterol concentrations (ID 4663), reduction of intestinal transit time (ID 4664) and reduction of inflammation (ID 1985) pursuant to Article 13(1) of Regulation (EC) No 1924/20061 EFSA Journal 9(6): 2214 Available at: Scientific Opinion on the substantiation of health claims related to chitosan and reduction in body weight (ID 679, 1499), maintenance of normal blood LDL-cholesterol concentrations (ID 4663), reduction of intestinal transit time (ID 4664) and reduction of inflammation (ID 1985) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 | EFSA (europa.eu).
EFSA (2020) Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 19(1):6343 Available at: Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283 | EFSA (europa.eu).
Fabec B., Hellstrom T., Henrysdotter G., et al. (2000). Active and intelligent food Packaging. A Nordic report on the legislative aspects. Nordic co-operation, p.29 Available at: Active and Intelligent Food Packaging: A Nordic Report on the Legislative ... - Google Books.
Fajardo P., Martins J.T., Fuciños C., et al. (2010) Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J. Food Eng. 101: 349-356.
Ferreira A.R., Alves V.D., & Coelhoso I.M. (2016) Polysaccharide-based membranes in food packaging applications. Membranes (Basel) 6:2.
FSANZ (2012) Supporting Document 1: Risk and Technical Assessment Report – Application A1077 Fungal Chitosan as a Processing Aid. Available at: A1077-ChitosanAppR-SD1.pdf (foodstandards.gov.au).
Gades M.D. & Stern J.S. (2003) Chitosan supplementation and fecal fat excretion in men. Obes Res. 11(5): 683-688.
Gades M.D. & Stern J.S. (2005) Chitosan supplementation and fat absorption in men and women. J Am Diet Assoc. 105(1): 72-77.
Garino C., Mielke H., Knüppel S., et al. (2020) Quantitative allergenicity risk assessment of food products containing yellow mealworm (Tenebrio molitor). Food and Chemical Toxicology 142: 111460.
Han W., Yu Y., Li J., et al. (2011). Application and safety assessment for nano-composite materials in food packaging. Chinese Science Bulletin 56(12): 1216-1225.
Hoff M., Trüeb R.M., Ballmer-Weber B.K., et al. (2003) Immediate-type hypersensitivity reaction to ingestion of mycoprotein (Quorn) in a patient allergic to molds caused by acidic ribosomal protein P2. J Allergy Clin Immunol. 111(5): 1106-10.
Huang B., Xiao D., Tan B., et al., (2016) Chitosanoligosaccharide reduces intestinal inflammation that involvescalcium-Sensing receptor (CaSR) activation in lipopolysaccharide(LPS)-challenged piglets. J. Agric. Food Chem. 64: 245-252.
Ifuku S. & H. Saimoto (2012) Chitin nanofibers: preparations, modifications, and applications. anoscale 4(11): 3308-18.
JFCRF (2011) Japan Food Chemical Research Foundation. List of Existing Food Additives. Available at: The Japan Food Chemical Research Foundation (ffcr.or.jp).
Jeevahan J. & Chandrasekaran M. (2019) Nanoedible films for food packaging: a review. J. Mater. Sci. 54: 12290-12318.
Kato Y., Yagami A., & Matsunaga K. (2005) A case of anaphylaxis caused by the health food chitosan. Arerugi 54: 1427-1429.
Kim D., Beck B.R., Heo S.B., et al. (2013) Lactococcuslactis BFE920 activates the innate immune system of olive flounder(Paralichthys olivaceus), resulting in protection against Streptococcus iniae infection and enhancing feed efficiency and weight gain in large-scale field studies. Fish. Shellfish Immunol. 35: 1585-1590.
Kim K.M., Son J.H., Kim S.K., et al. (2006). Properties of chitosan films as a function of pH and solvent type. Journal of Food Science 71(3): E119-E124.
Konovalova M.V., Markov P.A., Durnev E.A., et al. (2017) Preparation and biocompatibility evaluation of pectin and chitosan cryogels for biomedical application. J Biomed Mater Res. 105(2): 547-556.
Madhumathi K., Shalumon K.T., Rani V.V., et al. (2009) Wet chemical synthesis of chitosan hydrogel-hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol. 45(1): 12-15.
Moraru C., Mincea M.M., Frandes M., et al. (2018) A Meta-Analysis on Randomised Controlled Clinical Trials Evaluating the Effect of the Dietary Supplement Chitosan on Weight Loss, Lipid Parameters and Blood Pressure. Medicina 54: 109.
Nguyen M.X.H. (2012) Characterization of allergenic and antimicrobial properties of chitin and chitosan and formulation of chitosan-based edible film for instant food casing. Melbourne, Australia: Royal Melbourne Institute of Technology (RMIT) University, PhD thesis. Available at: 15625643.pdf (core.ac.uk).
NTP (2017) Technical Report on the Toxicity Study of Chitosan (CASRN 9012-76-4) Administered in Feed to Sprague Dawley [Crl:CD(SD)] Rats. Toxicity Report 93, National Toxicology Program, Public Health Service, U.S. Department of Health and Human Services.
Ouattara B., Simard R.E., Piette G., et al. (2000) Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int. J. Food Microbiol. 62: 139-148.
Pereira F., Pereira C., & Lacerda M.H. (1998). Contact dermatitis due to a cream containing chitin and a Carbitol. Contact Dermatitis 38(5): 290-291.
Pereira B., Venter C., Grundy J., et al. (2005) Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J. Allergy Clin. Immunol. 116: 884-892.
Qin C., Li H., Liu Y., et al. (2006) Water-solubility of chitosan and its antimicrobial activity. Carbohydrate Polymers 63: 367-374.
Qin Y., Zhang S., Yu J., et al. (2016) Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. Carbohydrate Polymers 147: 372-378.
Reese G., Ayuso R., & Lehrer S.B. (1999) Tropomyosin: An Invertebrate Pan-Allergen. International Archives of Allergy and Immunology 119(4): 247-258.
Remington B.C., Westerhout J., Meima M.Y., et al. (2020) Updated population minimal eliciting dose distributions for use in risk assessment of 14 priority food allergens. Food and Chemical Toxicology 139: 111259.
Romano P., Fabritius H., & Raabe D. (2007). The exoskeleton of the lobster Homarus americanus as an example of a smart anisotropic biological material. Acta Biomaterialia 3(3): 301-309.
Sánchez B., González-Tejedo C., Ruas-Madiedo P., et al. (2011) Lactobacillus plantarum extracellular chitin-binding protein and its role in the interaction between chitin, Caco-2 cells, and mucin. Appl. Environ.Microbiol. 77(3): 1123-6.
Singla A.K. & Chawla M. (2001) Chitosan: some pharmaceutical and biological aspects – an update. J Pharm Pharmacol 53: 1047-1067.
Sinha S., Tripathi P., & Chand S. (2012). A new bifunctional chitosanase enzyme from Streptomyces sp. and its application in production of antioxidant chitooligosaccharides. Applied Biochemistry and Biotechnology 167: 1029-1039.
Tapola N.S., Lyyra M.L., Kolehmainen R.M. et al. (2008) Safety aspects and cholesterol-lowering efficacy of chitosan tablets. J Am Coll Nutr. 27(1): 22-30.
Taylor G. & Wang N. (2018) Entomophagy and allergies: a study of the prevalence of entomophagy and related allergies in a population living in North-Eastern Thailand. Bioscience Horizons 11(8).
Tee R.D., Gordon D.J., Welch J.A., et al. (1993) Investigation of possible.
adverse allergic reactions to mycoprotein (‘Quorn’). Clin Exp Allergy 23: 257-60.
Tharanathan R.N. (2003). Biodegradable films and composite coatings: Past, present and future. Trends in Food Science and Technology 14(3): 71-78.
TNO (Netherlands Organisation for Applied Scientific Research) (2009). Repeated-dose (13-week) oral toxicity study in rats with chitin-glucan. Study Report provided to EFSA by Kitozyme.
Todorov S.D., Leblanc J.G., Franco B., et al., (2012) Evaluation of the probioticpotential and effect of encapsulation on survival for Lactobacillus plantarum ST16 Pa isolated from papaya. World J. Microbiol Biotechnol 28: 973-984.
UK NAC (2022) Website on Food allergies and Fungus. Accessed on 04/08/2022: Food allergies and Fungus - Aspergillosis Patients & Carers Support provided by the NHS National Aspergillosis Centre, UK
US FDA (2008) HemCon Notification. Available at: K080818.pdf (fda.gov).
US FDA (2012) Nutrition Center for Food Safety Applied. GRAS Notice Inventory - Agency Response Letter GRAS Notice No. GRN 000443. Available at: (accessed 01/08/2022) GRAS Notice 000443: Shrimp-derived chitosan (archive-it.org).
Van den Broek L.A.M., Knoop R.J.I, Kappen F.H.J., et al. (2015) Chitosan films and blends for packaging material. Carbohydrate Polymers 116: 237-242.
Vasile C. (2018) Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 11: 1834.
Waibel K.H., Haney B., Moore M., et al. (2011) Safety of chitosan bandages in shellfish allergic patients. Military Medicine 176: 1153-6.
Woo C.K. & Bahna S.L. (2011) Not all shellfish “allergy” is allergy! Clinical and Translational Allergy 1: 3.
Xia W., Liu P., Zhang J., et al. (2010). Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids 25: 170-179.
Yin M., Lin X., Ren T., et al. (2018) Cytocompatible quaternized carboxymethyl chitosan/poly(vinyl alcohol) blend film loaded copper for antibacterial application. Int J Biol Macromol 120: 992-998.
Young E., Stoneham M.D., Petruckevitch A., et al. (1994) A population study of food intolerance. Lancet 343: 1127-1130.
Zeng J.B., He Y.S., Li S.L., et al. (2012) Chitin whiskers: an overview. Biomacromolecules 13(1): 1-11.
Annex B to TOX/2022/45
Preliminary exposure calculations for chitosan-based BBFCMs (reserved)
This Annex is currently reserved as it contains unpublished data.
Secretariat
August 2022